
Li-Ion batteries are mainly 9 types according to its battery chemistry. Among them the most used Li-ion batteries are LiCoO2, NMC, LiFePO4, NCA, and LiPo. Each battery type has a specific energy density, thermal stability, chemical structure, durability, and safety. Therefore, these are used for specific applications.
The main 3 parts of the Li-ion batteries are anode, cathode and electrolyte medium. The anode and cathode are positive (+) and (-) terminals and the electrolyte medium is the filled battery chemistry material. The weight of the Li-ion battery is varied with the capacity, battery chemistry, and cell format and design. The most used 18650 cylindrical Li-ion cell weighs approximately 47 grams.
The Li-ion battery capacity is the storage of ampers inside the battery. It is measured in Ah. The battery capacity depends on electrolyte material, cell size, and the number of cells in the pack of Li-ion battery. The energy density of the Li-ion battery is the energy stored in the unit volume of the battery. Energy density mainly depends on the battery chemistry material.
The impedance of the Li-ion battery is the measurement of its resistance to the flow of electric current. Higher impedance can limit the battery’s effectiveness and reduce the overall lifespan. Lower impedance provides a higher discharge current when the Li-ion battery is used.
The charging voltage and current for of the Li- ion battery are 4.2V and 0.52A for the 3.7V cylindrical cell. Charging time for the 3.7V/cell Li-ion battery charge time is between 2-3 hours. Li-Ion batteries can be overcharged if it exceeds 4.2 V, and they can be overcharged if it is reduced to 2.5V.
Li-ion batteries have main 5 voltage states. Those are nominal voltage of 3.7/cell, charge cutoff voltage of 4.1-4.3V, end-of-discharge voltage of 2.5-3.0V, open circuit Voltage of 3.6-3.9V, and float voltage of 3.6-3.9V. These voltages are important while charging and discarding the li-ion battery. The standard discharge current of the Li-Ion 3.7/cell battery is between 0.2C and 1C.
The modern Li-ion battery memory effect is not considered. The self-discharge rate of Li-ion batteries is approximately 1.5-2% per month. But this can be varied with the temperature of the battery. Li-ion batteries can be used for approximately between 2-3 years or 300-500 charge cycles, whichever comes first. The cycle life of Li-Ion batteries is considered as 70-80% of the original capacity. The cycle life depends on battery material composition, charging, and operating conditions.
Li-ion batteries should be used in the proper temperature. For the charging, use 0°C to 45°C. You can use it between -20°C to 60°C and it should store 15°C +/- 5°C when it is not in use. Li-ion batteries can be damaged due to overcharging, deep discharging, heating, cooling, physical damages, incorrect storage, water, vapour, inappropriate chargers, and not used in a long time mainly.
Due to the higher energy density, lower self-discharge, small size, no memory loss Li-Ion batteries are used in a wide range of applications such as small consumer products, vehicles and power sources mainly.
What are the 9 Types of Li-Ion Batteries?

Li-ion batteries are of various types according to its applications and the materials that are used in the cathode. Each Li-Ion battery type has a specific energy density, thermal stability, chemical structure, durability, and safety. Therefore, each type is used for specific tasks. The most used Li-ion batteries are LiCoO2, NMC, LiFePO4, NCA, and LiPo. There are 9 main types of Li-ion batteries are available for different purposes with the above features.
- Lithium Cobalt Oxide (LiCoO2): The most common type, with high energy density but risk of thermal runaway. Used in mobile phones, tablets, and laptops.
- Lithium Manganese Oxide (LiMn2O4): Lower energy density but better safety than LiCoO2. Used in medical devices, power tools.
- Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2 or NMC): High energy density, good thermal stability. Used in electric vehicles and power tools.
- Lithium Iron Phosphate (LiFePO4): Lower energy density but safer chemical structure and longer life. Used in electric vehicles and power tools.
- Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2 or NCA): High energy density but less stable. Used in electric vehicles, laptops, and mobile devices.
- Lithium Titanate (Li2TiO3): Fast charging ability, long life, safe but lower energy density. Used in electric vehicles, and grid storage.
- Lithium Polymer (LiPo): Flexible form factor but requires protective circuitry. Used in mobile devices, and wearables.
- Solid State Batteries: Still in the R&D phase but expected to have higher energy density and safety.
- Lithium Nickel Manganese Oxide (LiNiMnO2 or LNMO): Offers high power and safety compared to LiCoO2, used in power tools and medical devices.
What are the Main 8 Parts of the Li-Ion Battery?
Li-Ion battery has a main 8 parts. Each part has manufactured with different types of material with specific tasks. Outer visibility parts are the anode, cathode, and cell casing. The inner parts are electrolyte, separator, current collectors, binders and cathode and anode tabs. Below are the main 8 parts, tasks and the used material for the specific part.
- Anode – The negative electrode, usually made of graphite or silicon. This is where the lithium ions are stored when the battery is charged.
- Cathode – The positive electrode, made of a lithium metal oxide like lithium cobalt oxide (LiCoO2) or lithium iron phosphate (LiFePO4). This provides the lithium ions that migrate to the anode.
- Electrolyte – The medium that allows lithium ions to flow between the cathode and anode. Usually, a lithium salt like LiPF6 is dissolved in organic solvents.
- Separator – A permeable membrane, usually made of polyolefins, that prevents electrical contact between the anode and cathode while allowing lithium ions to pass through.
- Current collectors – Thin metal foils, usually copper for the anode and aluminium for the cathode, that collect and transfer electrical current.
- Binders – Adhesives like polyvinylidene fluoride (PVDF) that hold active cathode/anode materials to the current collectors.
- Anode/Cathode tabs – Electrical contacts that connect each electrode to the external circuit.
- Cell casing – The external housing, often made of rigid metal like steel or aluminium, that contains all the internal battery components.
What is the Weight of a Li-Ion Battery???
The most used 18650 cylindrical Li-ion cell weighs approximately 47 grams. The weight of a lithium-ion battery is varied with its capacity, chemistry, cell format and design.
- Battery Capacity: Larger Li-ion batteries are more weight due to the inclusion of active material inside the battery.
- Cell Chemistry: Different cell chemistries have different energy densities. Therefore Lithium Cobalt Oxide (LCO) and Lithium Nickel Manganese Cobalt Oxide (NMC) are high energy density materials; therefore, the battery weight is light. But Lithium Iron Phosphate (LFP) and Lithium Titanate (LTO) will be more weight due its low energy density.
- Cell Format: Battery formats like cylindrical, prismatic, and pouch have different space efficiencies, affecting weight. Cylindrical cells, while robust, don’t use space as efficiently as prismatic or pouch cells.
- Cell Format: Different cell formats of Li-ion batteries are available for various uses. The most used cell formats are cylindrical, prismatic, and pouch. These
- Packaging: The weight can also be influenced by the choice of packaging materials and their design, which need to provide structural integrity and environmental protection.
The weight of the Li-Ion battery is measured in kilograms (kg) or grams (g). The small Li-ion batteries that are used in consumer electronics devices can weigh between 50 grams to 300 grams. Mid-size range Li-ion battery packs can weigh between 2 kg – 5 kg. Larger Li-ion batteries which are used in EVs can weigh between 100kg -500kg.
What is the Capacity of a Li-ion Battery?
The capacity of a lithium-ion battery is defined as how much electric charge it can deliver in an hour. The small battery capacity is measured in milliampere-hours (mAh) and the large battery capacity is measured in ampere-hours (Ah). Battery capacity depends on the electrode materials, cell size, and number of cells in a pack.
Below are the most used Li-ion batteries and their capacities.
- Li-ion Coin cells and mini cylindrical cells – <100 mAh
- Li-ion Smartphone batteries – 2,000-4,000 mAh
- Li-ion Vaping/e-cig batteries – 1,000-3,000 mAh
- Li-ion Laptop batteries – 4,000-12,000 mAh
- Power tool batteries Li-ion – 1-5 Ah
- Li-ion Electric bicycle batteries – 6-15 Ah
- Li-ion Electric motorcycle/scooter – 10-60 Ah
- Li-ion Electric cars – 50-100 kWh (equivalent to 50,000-100,000 Ah)
What is the Energy Density of a Li-Ion Battery?
The energy density of a lithium-ion battery is the amount of energy stored per unit volume or weight. The higher energy density is more energy stored in a considered place. A battery can be used for longer runtime and range if it has a higher energy density.
The highest energy density Li-Ion battery is lithium-cobalt-oxide (LCO). The lower energy density Li-ion battery is lithium-iron-phosphate (LFP). The energy density is measured in Watt-hours/litre (Wh/L) or Watt-hours/kilogram (Wh/kg).
The energy density of Li-Ion batteries in different applications
- Cylindrical 18650 cells – 240-265 Wh/kg, 500-700 Wh/L
- Prismatic EV cells – 130-200 Wh/kg, 250-400 Wh/L
- Smartphone battery – 100-265 Wh/kg, 300-750 Wh/L
- Laptop battery – 80-190 Wh/kg, 250-670 Wh/L
- Electric bicycle battery – 90-140 Wh/kg
- Electric car battery pack – 100-250 Wh/kg, 100-400 Wh/L
What is the Impedance of the Li-ion Battery??

The impedance of a lithium-ion battery is a measurement of its resistance to the flow of electric current. Impedance is an important factor in determining the battery’s efficiency and performance. The Li-ion battery impedance is mainly 4 types. These are Ohmic resistance, charger transfer resistance, diffusion resistance, and inductive resistance.
- Ohmic resistance – Resistance to ion flow through the electrolyte. Typically 50-200 m Ohm for Li-ion cells.
- Charge transfer resistance – Resistance as ions transfer between electrodes, typically 50-500 m Ohm.
- Diffusion resistance – Ion diffusion into electrodes, around 100-300 mOhm.
- Inductive reactance – Opposition from cell components to changing current, typically 5-50 μH.
High impedance can limit the battery’s effectiveness and reduce its overall lifespan.
Lower impedance allows a battery to provide higher discharge currents and power delivery.
So total Li-ion cell impedance ranges from around 100-1000 milliohms.
What is the Charging Voltage of Li-ion Battery?
The most used 18650 cylindrical Li-ion cell requires to charge with a constant voltage up to 4.2V and a constant current between 0.5C and 1C (where 1C is the rated capacity). The charging voltage can be varied with the battery connection and battery chemistry.
Li-ion batteries can be connected in serial and parallel. The serial connection system requires a higher charging voltage. In the parallel connection, it requires a lower charging voltage than a serial connection.
Li-ion batteries have different types of battery chemistries. Therefore, the charging voltage can vary. For example, Lithium Iron Phosphate (LiFePO4) batteries have a lower charging voltage, typically around 3.6V per cell.
What is the Charging Time of a Li-Ion Battery?
The most used 3.7V/cell Li-ion battery charge time is between 2-3 hours. Li-ion battery charging time can be varied with these factors such as battery capacity, charging current, charging voltage, charging power, battery temperature and battery chemistry.
- Battery capacity – Battery capacity is measured in mAh or Ah. Higher capacity batteries take longer to charge fully.
- Charging current – Charging current is measured in Amps. Higher charger current reduces charging time.
- Charging voltage – Constant-voltage charging up to 4.2V per cell.
- Charger power – Charging current x voltage. Higher charger power enables faster charging.
- Temperature – Charging is slower at low temps and faster at higher temps (up to 45°C).
Read More About – Cordless Drill Battery Charge Time – (08 Brands Tested)
What is Overcharging and Over Discharging of Li-ion Batteries?
Overcharging
Overcharging a battery is defined as charging a battery beyond its maximum 4.2V limit. Overcharging happens when a charger doesn’t cut off the power supply to the battery once it has reached its maximum charge. This is harmful because it can cause excess heat to build, lead to possible leaks, the battery swelling or even exploding. Overcharging can also significantly shorten the battery’s lifespan.
Over discharging
The over-discharging battery is defined as the battery being drained to a 2.5 voltage limit. This is known as the deep discharge of the battery. Over-discharging can cause a change in the structure of the battery’s cells, making them unusable or greatly reducing their capacity and lifespan. It can also result in a phenomenon known as a “zero-volt condition,” in which the battery’s voltage drops so low that it cannot be recharged.
What are the Voltages in Li-Ion Battery?
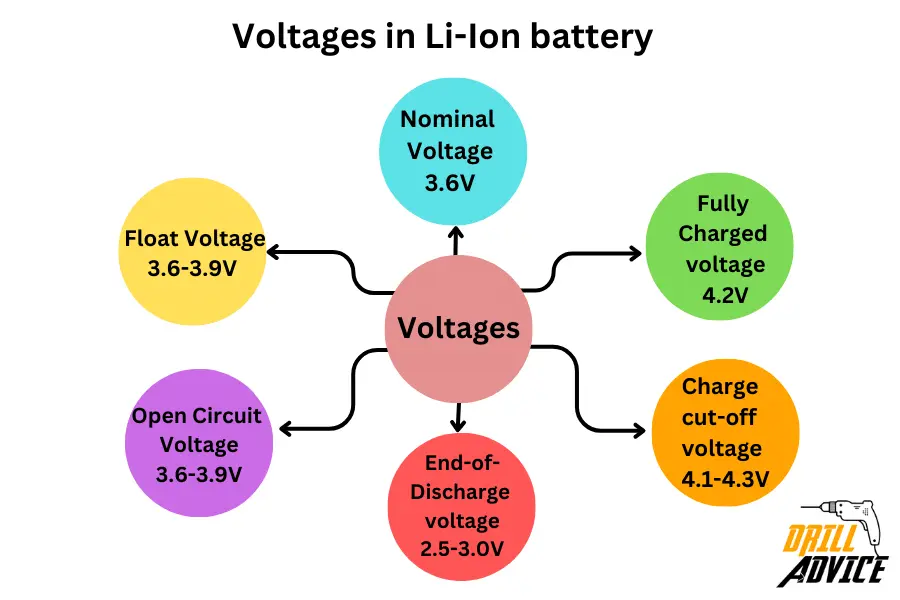
The voltage levels of Li-Ion batteries have different values. Each of voltage level affect the battery performance and battery life directly. The most important voltages in the Li-ion battery are nominal voltage, fully charged voltage, charge cutoff voltage, end-of-discharge voltage, open circuit voltage, and float voltage. Each voltage have specific values and limits. Those are;
- Nominal Voltage: Li-ion cells are typically named based on their nominal voltage
- Fully Charged voltage is the maximum voltage per cell during charging. 4.2V is the standard charge voltage for Li-ion batteries.
- The charge cut-off voltage is 4.1-4.3V. After the cutoff voltage, charging is stopped once the cell voltage reaches the cut-off level to prevent overcharging.
- End-of-Discharge voltage is 2.5-3.0V. It is stopped once the cell voltage drops to this level to prevent over-discharging.
- Open Circuit Voltage is 3.6-3.9V. The open circuit voltage is the unused/unconnected voltage of a cell. It can drop over time due to self-discharge.
- The float Voltage is 3.6-3.9V. The float voltage is used when a cell is fully charged but not in active use. Prevents self-discharge.
What is the Nominal Voltage of a Li-Ion Battery?
The nominal voltage of a lithium-ion battery is the approximate average voltage that the cell maintains during the regular discharge operation. For lithium-ion batteries, the nominal voltage is typically 3.6 or 3.7 volts per cell. This means that a lithium-ion cell with a nominal voltage of 3.6V will operate around 3.6V on average as it discharges from full (4.2V) down to the minimum cutoff voltage (2.5-3V).
The nominal voltage is different from the charged voltage (4.2V) or end-of-discharge voltage (2.5-3V). It is the mid-point voltage during normal operation. For example, a 18650 lithium-ion cell, one of the most common sizes, is named based on its dimensions (18mm diameter, 65mm height) and its 3.6/3.7V nominal voltage. The nominal voltage is useful for estimating the operation and performance of lithium-ion batteries in devices and applications. It also determines the number of cells needed to achieve a desired pack voltage since cells in series sum their voltages.
What is the Discharge Voltage of Li-ion Battery?
The discharge voltage of a lithium-ion battery is the voltage of the battery during the process of discharging or using the battery to power a device/load. When a lithium-ion cell is used to drive a load, its voltage will decrease from the fully charged voltage of 4.2V down towards the cut-off/minimum discharge voltage, which is typically 2.5-3V per cell.
Discharge voltage depends on these 4 factors such as;
- Battery’s internal resistance
- Discharge current (higher currents cause faster voltage drop)
- State of charge (voltage drops more rapidly at the end of discharge)
- Temperature (lower temps increase internal resistance and voltage drop)
The discharge voltage profile steadily decreases over time, starting from around 3.8-4V on a full battery down to the cut-off of around 2.5-3V. The average discharge voltage during normal operation is a nominal voltage of 3.6/3.7V. Battery management systems monitor discharge voltage to prevent over-discharging, which can damage the battery.
What is the Nominal Current of Li-ion Battery?
The nominal current of a lithium-ion battery is the maximum recommended continuous current that the battery can safely provide during discharge or charging. The nominal current is measured in amperes (A) or milliamperes (mA). The nominal current represents the optimal, normal operating current for the battery in terms of performance and longevity.
Exceeding the nominal current can potentially damage a Li-ion battery through overheating, lithium plating, or loss of capacity.
What is the Charging Current of a Li-ion Battery?
The standard charge current for lithium-ion batteries is the recommended optimal current for charging the battery under normal conditions according to international standards. The standard charge current is typically 0.5C to 1C (where 1C is the rated capacity of a cell in amp-hours).
Charging at 0.5C to 1C represents a relatively slow to moderate charge rate that maximizes battery lifetime and provides a balance between charge time and longevity. Faster charging above 1C current can be done occasionally, but frequent fast charging will degrade the battery more quickly over time. The maximum charge current is usually 4C, beyond which safety and battery health are compromised.
Some effects of the standard 1C charge current include:
- Charge time – It takes about 1-2 hours for a full charge (depending on capacity)
- Heat generation – Low heating lowers ageing effects
- Side reactions – Minimizes undesirable chemical reactions
- Internal stress – Reduces mechanical strain on battery components
- Cycle life – Optimizes battery lifespan by limiting damage accumulated per charge cycle.
What is the Standard Discharge Current of Li-ion Battery?
The standard discharge current for a lithium-ion battery is the recommended maximum continuous current that the battery can safely supply during normal discharging conditions according to international standards. The standard discharge current is typically defined based on the C-rate, similar to charging.
For lithium-ion batteries, the standard discharge current is usually between 0.2C and 1C. For example, a 1000 mAh Li-ion cell would have a standard discharge current of 200 mA to 1000 mA. Discharging at 0.2C to 1C represents a moderate load that balances performance with battery lifetime and heat generation.
Effects of the standard discharge current:
- Allows the battery to operate within safe thermal limits for everyday use. Excessive currents can overheat the battery.
- Causes minimal voltage drop or sag, maintaining optimal operating voltage.
- Results in efficient energy delivery and conversion during discharge.
- Limits mechanical and chemical stresses on battery components.
- Enables the optimal lifetime in terms of achievable cycles before capacity fades.
- Allows for temporary surge currents above 1C for short periods if needed.
What is the Memory Effect of Li-ion Battery?
The memory effect is the phenomenon where a battery seems to ‘remember’ its previous low state of charge and cannot deliver its full rated capacity. The memory effect does not apply to modern lithium-ion batteries. Li-ion batteries have no known memory effect.
Li-ion batteries do not need to be periodically discharged fully before recharging. They have no memory of previous partial discharge cycles. This makes lithium-ion batteries more convenient to use than old NiCd batteries.
There is no need for occasional full discharge cycles. However, for maximum lithium-ion battery lifespan, it is still advisable to avoid frequently recharging from a partially charged state. Still allowing occasional full discharges helps maintain internal battery balance. But this is not due to any specific memory effect in lithium-ion batteries. They can generally be recharged as needed without capacity loss or ill effects.
What is the Discharge Capacity of Li-ion Battery?
The discharge capacity of a lithium-ion battery is the amount of electric charge that can be drawn from a fully charged battery until it reaches the minimum cut-off voltage. It represents the usable electrical energy stored in the battery.
The discharge capacity is measured in ampere-hours (Ah) or milliampere-hours (mAh). A Li-ion cell’s discharge capacity depends on its cathode chemistry, anode material, electrolyte, and design factors. The discharge capacity diminishes as a battery age after repeated charge/discharge cycles due to the loss of active lithium ions.
Battery protection circuits stop discharge once the cut-off voltage, like 3V per cell, is reached to prevent damage from over-discharging.
What is the Self-Discharge Rate of a Li-ion Battery?
For Li-ion batteries, the self-discharge rate is approximately 1.5-2% per month but it can be higher if the battery is stored at higher temperatures. This is relatively low compared to other types of rechargeable batteries, such as Nickel-Cadmium (NiCd) or Nickel-Metal Hydride (NiMH) batteries, which can have self-discharge rates as high as 10-15% per month.
The self-discharge rate of a battery is a measure of how much it will discharge (lose its charge) when not in use. All batteries, including Lithium-ion (Li-ion) batteries, have some level of self-discharge, but the rate can vary depending on the type of battery and the conditions it’s stored in.
The self-discharge rate is an important factor to consider when storing batteries for long periods. Even if a battery is not being used, it will slowly lose its charge over time, which can lead to a situation where the battery is dead or has a significantly reduced charge when you need to use it.
What is the Lifespan of a Li-Ion battery?
The lifespan of the Li-ion battery is approximately between 2-3 years or 300-500 charge cycles, whichever happens first. The lifespan of the Li-ion battery varied with the charge/discharge cycles, depth of discharge, temperature, overcharging/over-discharging, and battery management system (BMS).
- Charge/Discharge Cycles: The life of a Li-ion battery is often defined in terms of charge/discharge cycles. A cycle is defined as the process of charging a battery from 0 to 100% and then discharging it from 100% back to 0%. Most Li-ion batteries can handle 300 to 500 cycles before their capacity drops to 80% of the original capacity. However, the actual number can vary greatly depending on other factors.
- Depth of Discharge (DoD): Deeper discharges put more strain on the battery and reduce its lifespan. In other words, a battery that is regularly discharged to 50% will last longer than one that is regularly discharged to 10%.
- Temperature: High temperatures can greatly reduce the life of a Li-ion battery. Heat causes the electrolyte inside the battery to break down faster, which can lead to reduced capacity and, ultimately battery failure.
- Overcharging/Over-discharging: Overcharging or over-discharging a battery can also reduce its lifespan. Most modern devices and chargers are designed to prevent this from happening, but it’s still a potential issue, especially with older or cheaper devices.
- Battery Management System (BMS): A good BMS can help to prolong the life of a Li-ion battery by protecting it from overcharge, over-discharge, short circuit, and overheating.
What is the Cycle Life of a Li-ion Battery?
The cycle life of a lithium-ion battery is the number of complete charge-discharge cycles the battery can undergo before its capacity degrades below usable levels. The cycle life is considered as 70-80% of original capacity. The cycle life depends on battery material composition, charging practices, and operating conditions.
3 main Importance of cycle life.
- Determines battery lifespan and replacement needs – higher is better
- Affects the lifetime operating costs of battery-powered devices and vehicles
- Influences design tradeoffs between energy density and longevity
5 ways to maximise cycle life
- Use within operating voltage and current limits
- Prevent excessive heat buildup during use
- Avoid fully charging or discharging regularly
- Use moderate charge and discharge rates
- Keep cells balanced in multi-cell packs
What are the Best Temperature for Li-ion Batteries?
The temperature of the Li-ion battery is very important for higher performance, safety and a longer lifespan. Therefore Li-ion battery should charge between 0°C to 45°C. It can use between -20°C to 60°C. It should store 15°C +/- 5°C when it is not in use.
Effects of temperature:
- Performance – Increasing temperature speeds up lithium ion mobility, improving conductivity and capacity. But only to a point.
- Safety – High temperatures above 50°C increase risk of overheating and thermal runaway. Low temperatures can cause lithium plating if charged.
- Lifespan – High temperatures accelerate side reactions and component degradation. But low temperatures reduce capacity and cycle life.
- Charging – 0-45°C allows efficient absorption and intercalation of lithium ions into the anode. Outside this range causes capacity loss.
- Discharging – Discharge capacity is reduced at very cold temperatures but usable down to -20°C. High temperatures above 60°C shorten cell life.
- Storage – Storing cells around 15°C with low swing minimizes self-discharge and degradation while inactive.
How Can a Li-ion Battery Get Damaged?
Li-ion batteries can damage due to overcharging, deep discharging, heating, cooling, physical damages, in correct storage, water, vapour, inappropriate chargers, and not being used in a long time mainly.
Overcharging: Li-ion batteries can overcharger when a battery is left to charge beyond its maximum capacity. This can lead to excessive heat and pressure inside the battery, which can cause damage and reduce the battery’s lifespan.
Deep Discharge: Li-ion batteries can deeply discharge when a battery’s charge is allowed to run down too low or even completely. Li-ion batteries have a cutoff voltage to avoid this, but if this mechanism fails or if the battery is not in use for an extended period, it can discharge below the safe level, causing irreversible damage.
Heat: High temperatures can accelerate chemical reactions inside the Li-ion battery. Heating can lead to faster degradation of the battery’s capacity. It’s always a good idea to store and use batteries in cool and well-ventilated environments.
Cold: Extremely cold temperatures can harm Li-ion batteries. Low temperatures can cause the electrolyte inside the batteries to crystallize, leading to internal short circuits.
Physical Damage: This includes anything from dropping the battery to puncturing it. Physical damage can cause internal short circuits, leaks, or even fires in severe cases.
Incorrect Storage: If you’re not using a Li-ion battery for a while, it’s best to store it at about 40-60% charge. Storing a battery at full charge for extended periods can stress the battery and reduce its overall lifespan.
Using Non-standard Chargers: Not all chargers are created equal, and using a charger that’s not suited to your battery can lead to overcharging or faster wear and tear.
What are the Applications of Li-Ion Batteries?

Lithium-ion batteries are used in many applications due to their high energy density, low self-discharge rate, and lack of memory effect. Some of the most common applications of Li-ion batteries are;
- Consumer electronics – Cell phones, laptops, tablets, digital cameras etc. Li-ion batteries allow designing slim and compact devices.
- Electric vehicles – Cars, bikes, buses, boats. Li-ion offers high energy density critical for long-range and performance.
- Aerospace – Li-ion batteries are used to power spacecraft systems and in jet engines to start auxiliary units. Lightweight is beneficial.
- Medical devices – Implantable medical devices, mobility aids etc. Li-ion provides compact power.
- Power tools – Drills, saws, garden equipment, and other cordless power tools leverage Li-ion batteries for untethered usage.
- Energy storage – Li-ion battery banks help store and smooth renewable energy, provide backup power.
- Military applications – Li-ion batteries power communications, night vision goggles, laser systems used in the defence sector.
- Vaping devices – Popular choice for vapes and e-cigarettes due to high energy density and safety.
What are the 10 Advantages of Li-ion Battery?
- High energy density – Li-ion packs more energy per unit volume and weight, enabling compact, light devices.
- High power density – Capable of providing high surge currents and power output.
- Low self-discharge – Stores charge for longer durations with less than 5% loss per month.
- No memory effect – Can be partially recharged without reducing usable capacity.
- Low maintenance – Does not require scheduled cycling or full discharges to maintain performance.
- High efficiency – Up to 99% efficiency in charge-discharge cycles. More of the supplied energy is usable.
- Good high-temperature performance – Can operate at temperatures up to 60°C though best below 40°C.
- Flexible form factors – Available in cylindrical, prismatic and pouch designs to suit different applications.
- Faster charging – Can be rapidly charged within 1-3 hours for consumer cells using fast charging techniques.
- High voltage – Operates safely at up to 4.2V per cell relative to 1.2-2V for other chemistries.
Read More About – What to Know About Ni-Cd Battery?
