
Cobalt is a ferromagnetic metal with a specific gravity of 8.9. Cobalt is a harder and tougher metal with a Mohs hardness of 5. Cobalt is resistant to corrosion and higher heat. Hence cobalt is used to make alloys with high corrosion resistance and thermal stability tools.
Cobalt has physical and chemical properties. In room temperature, cobalt is a solid metal, and it does not react with water, acid and air. But the physical and chemical properties will be varied in higher temperatures. Cobalt is mixed with chromium, molybdenum, nickel, tungsten, vitallium, and aluminum due to the hardness and corrosion resistance of the cobalt. These Alloys are used for power tools, the aerospace industry, the health industry and the mining industry mainly.
M35 and M45 are the most used cobalt metals which are used for the power tool manufacturing industry. Cobalt has a unique blue color; therefore, it has been used as jewelry. As a result of this, cobalt has a long historical evolution since the third millennium.
What are the Chemical Properties of Cobalt?
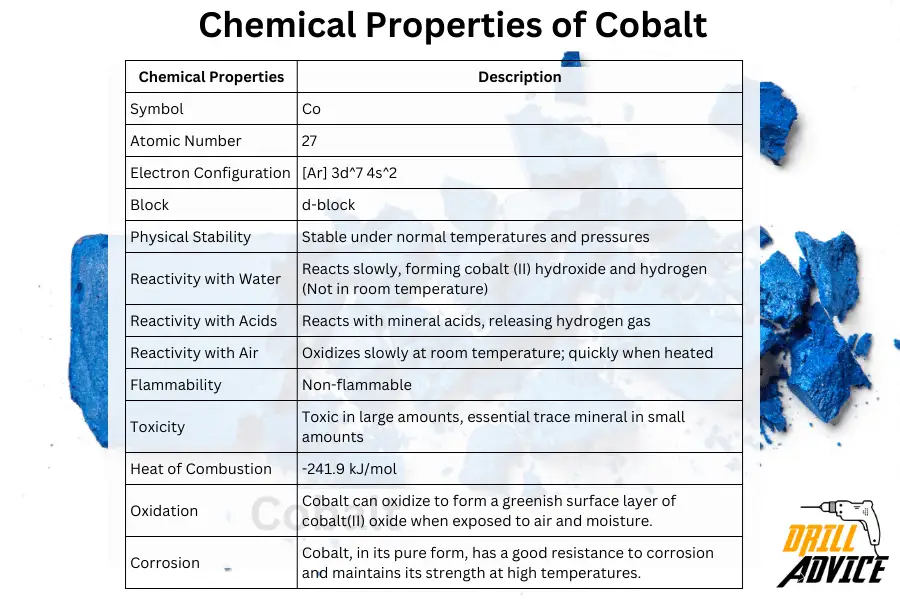
Chemical properties can be considered as the properties which are changed before or after the chemical reactions. Physical stability, water, air and acid reactivities are the most important chemical properties of the coablt. The most important chemical properties of cobalt are corrosion, corrosion resistance and oxidation.
These are the chemical properties of cobalt and its values.
| Chemical Properties | Description |
|---|---|
| Symbol | Co |
| Atomic Number | 27 |
| Electron Configuration | [Ar] 3d^7 4s^2 |
| Block | d-block |
| Physical Stability | Stable under normal temperatures and pressures |
| Reactivity with Water | Reacts slowly, forming cobalt (II) hydroxide and hydrogen (Not in room temperature) |
| Reactivity with Acids | Reacts with mineral acids, releasing hydrogen gas |
| Reactivity with Air | Oxidizes slowly at room temperature; quickly when heated |
| Flammability | Non-flammable |
| Toxicity | Toxic in large amounts, essential trace mineral in small amounts |
| Heat of Combustion | -241.9 kJ/mol |
| Oxidation | Cobalt can oxidize to form a greenish surface layer of cobalt(II) oxide when exposed to air and moisture. |
| Corrosion | Cobalt, in its pure form, has a good resistance to corrosion and maintains its strength at high temperatures. |
What are the Physical Properties of Cobalt?
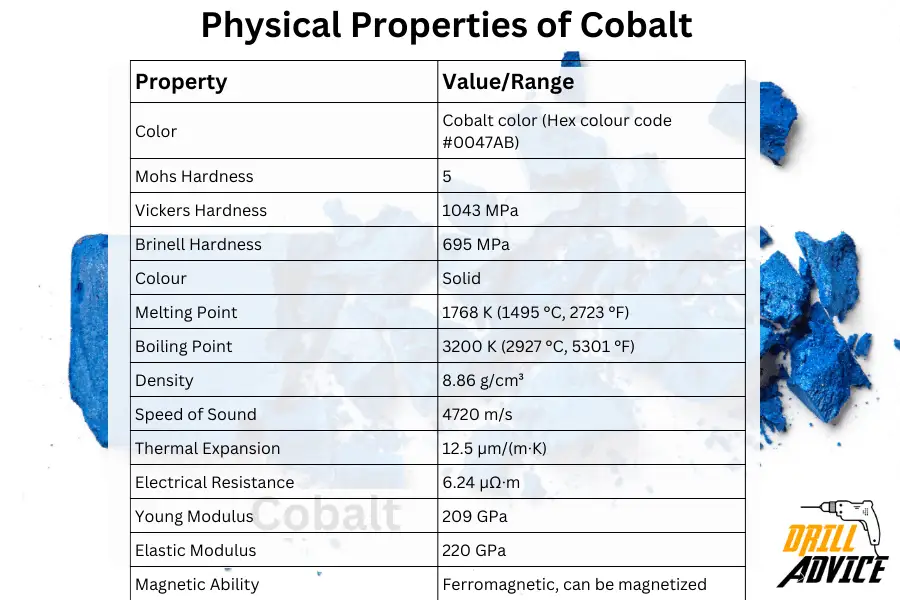
Physical properties characterize how a material behaves under various conditions without altering its chemical composition. Physical properties are important when the cobalt is used for various purpose. Physical properties of cobalt can be varied with the using temperature. The most important physical properties of cobalt are hardness, melting point, boiling point, density, speed of sound, thermal expansion, electrical resistance, young modulus, and elastic modulus.
The below table includes each property and relevant values and ranges for the physical properties of the cobalt.
| Property | Value/Range |
|---|---|
| Color | Cobalt color (Hex colour code #0047AB) |
| Mohs Hardness | 5 |
| Vickers Hardness | 1043 MPa |
| Brinell Hardness | 695 MPa |
| Colour | Solid |
| Melting Point | 1768 K (1495 °C, 2723 °F) |
| Boiling Point | 3200 K (2927 °C, 5301 °F) |
| Density | 8.86 g/cm³ |
| Speed of Sound | 4720 m/s |
| Thermal Expansion | 12.5 µm/(m·K) |
| Electrical Resistance | 6.24 µΩ·m |
| Young Modulus | 209 GPa |
| Elastic Modulus | 220 GPa |
| Magnetic Ability | Ferromagnetic, can be magnetized |
What are the Usage of Cobalt?
Cobalt has a higher hardness. Therefore cobalt is mixed with other metals to produce the alloys. The hardness of the alloy depends on the cobalt percentage. Therefore, cobalt is widely used for drill bits, saw blades. But cobalt is mostly used in many industrial applications.
Primary usage:
- Manufacturing sturdy drilling and cutting tools, due to its superior hardness.
- Woodworking and DIY projects: Cobalt tools resist wear and tear over prolonged use and provide precision and longevity.
Other industrial applications:
- Aerospace industry: Cobalt’s heat-resistant nature is used in the manufacturing of jet engines and gas turbines.
- Cobalt is used to make superalloys. These superalloys are higher heat resistance and corrosion.
- Health sector: Cobalt’s strength and durability make it suitable for the creation of orthopaedic implants.
- Electronics: Cobalt is an essential component in rechargeable batteries, enabling efficient energy storage and release.
- Glass and Ceramics industry: Cobalt imparts a brilliant blue colour.
- Petroleum and Chemical Industries: Cobalt is used as a catalyst to facilitate various chemical reactions.
- Automotive Industry: Cobalt is used in the production of rechargeable batteries, which are widely used in electric vehicles.
- Paint Industry: Cobalt salts are used in the production of pigments that provide a distinct blue color to paints and dyes.
- Radiotherapy: The radioisotope cobalt-60 is used in medical treatments, such as in radiation therapy to treat cancer.
- Magnetic Alloys: Cobalt is used to create powerful magnets used in motors, generators, and magnetic resonance imaging (MRI) machines.
- Metallurgy: Cobalt is used in superalloys that retain strength at high temperatures, perfect for turbine blades for jet engines, gas turbines, and even rocket engines.
- Animal Nutrition: Cobalt is a crucial component of Vitamin B12, thus used as a dietary supplement in animal feed.
- Radioactive Tracing: Radioactive isotopes of cobalt are used for tracing in geochemical and industrial applications.
- Catalysts: Cobalt is utilized as a catalyst in the Fischer-Tropsch process for liquid fuel production from syngas.
These examples illustrate the versatility of cobalt and its importance across various industries.
What are the Cobalt Alloys and Mixed Metals?
Cobalt alloys are manufactured by mixing cobalt, chromium, molybdenum, nickel, tungsten, vitallium, and aluminum. The Cobalt alloys are resistant to wear, corrosion, and high temperature. But these are higher strength and lightweight. Therefore these are used for many applications.
- Stellite Alloys: Composed predominantly of cobalt, chromium, and tungsten, Stellite alloys are famed for their resistance to wear, corrosion, and high temperatures. Often used in cutting tools and engine valves.
- MP35N: This is a multi-phase alloy containing nickel, cobalt, chromium, and molybdenum. It possesses ultra-high strength and corrosion resistance, making it a popular choice in biomedical implants and aerospace applications.
- Superalloys: Cobalt is a critical component in superalloys, primarily used in aircraft engine parts. In these alloys, cobalt is mixed with nickel, chromium, tungsten, and sometimes, a small quantity of iron.
- HS-25: An iron-nickel-cobalt alloy, HS-25, is resistant to oxidation and thermal fatigue, often used in gas turbine engine parts.
- Vitallium: An alloy of cobalt, chromium, and molybdenum, Vitallium is used in making dental and orthopedic implants due to its bio-compatibility.
- Alnico: A combination of aluminum, nickel, and cobalt, Alnico forms strong permanent magnets.
- L-605: This alloy comprises cobalt, chromium, tungsten, and nickel, offering great formability and high-temperature strength, perfect for applications like gas turbine technology.
Read More About – Know about Cast Iron: Properties, Production, Types, Uses, Pros and Cons
What are M35 and M42 Cobalt?
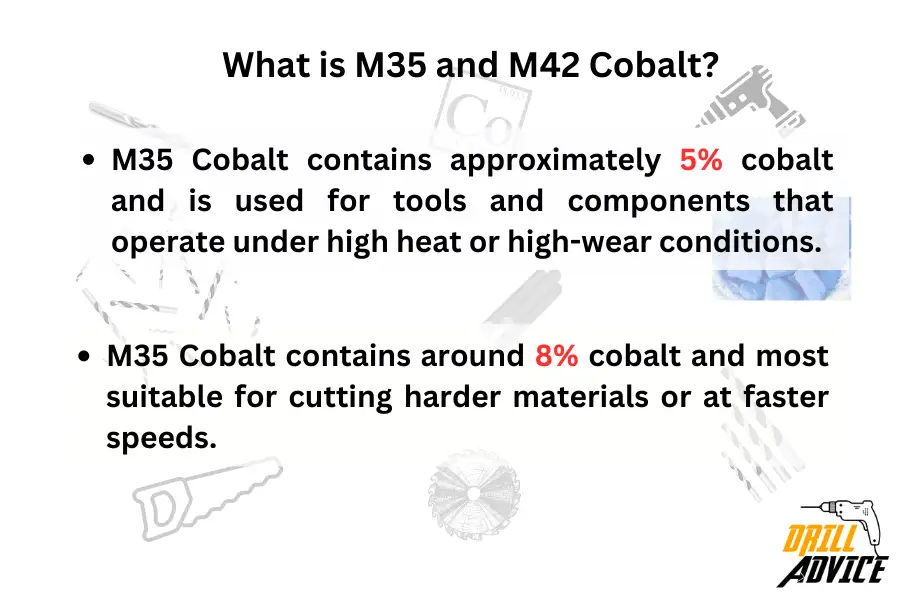
Cobalt can be mixed with . According to the percentage of cobalt, mixture properties will be changed. The M35 is named as 5% cobalt included with HSS, and M42 is named as 8% cobalt included HSS metal.
- M35 Cobalt: M35 is known as a cobalt high-speed steel (HSS). It contains approximately 5% cobalt and is renowned for its ability to maintain hardness at high temperatures. Because of this, M35 is often used in tools and components that operate under high heat or high-wear conditions.
- M42 Cobalt: M42 is another cobalt high-speed steel with a higher cobalt content than M35, usually around 8%. The increased cobalt content gives M42 even greater hardness and heat resistance than M35, making it ideal for more demanding applications like cutting harder materials or at faster speeds.
Read More About – M35 and M42 Cobalt Drill Bits: Differences and Usage
History of Cobalt
Cobalt is an ancient metal. Cobalt is used in history due to its bright blue color. It has used for jewelry and glass manufacturing since the Third millennium. These are years and evolutions of the cobalt usage.
- Third millennium BC: Cobalt detected in Persian jewelry.
- 14th century BC: Cobalt was used to color glass during the Bronze Age. Evidence from the Uluburun shipwreck.
- 1550–1292 BC: The oldest cobalt-colored glass found from the eighteenth dynasty of Egypt.
- 79 AD: Cobalt found in the ruins of Pompeii.
- 618–907 AD: Cobalt used during China’s Tang dynasty.
- 1368–1644 AD: Cobalt usage continued into China’s Ming dynasty.
- 16th century: The first mines for the production of smalt (cobalt glass powdered for use as pigment) located in Norway, Sweden, Saxony, and Hungary.
- 1735: Swedish chemist Georg Brandt credited with discovering cobalt, distinguishing it from other traditional metals.
- 19th century: A significant part of the world’s cobalt blue and smalt production was carried out at the Norwegian Blaafarveværket.
- 1864: Discovery of cobalt ore in New Caledonia, leading to a decline in European cobalt mining.
- 1904: Discovery of cobalt ore deposits in Ontario, Canada.
- 1914: Discovery of even larger cobalt deposits in the Katanga Province in the Congo.
- 1938: Discovery of the radioisotope cobalt-60 by John Livingood and Glenn T. Seaborg.
- 1950: Cobalt-60 used at Columbia University to establish parity violation in radioactive beta decay.
- After World War II: The US prospected for cobalt domestically, finding an adequate supply in Idaho near Blackbird canyon.
- 1978: Onset of the Shaba conflict; the copper mines of Katanga Province nearly ceased production.
- Present: Cobalt is being discussed as a critical component in a world running on renewable energy and dependent on batteries.
