
Titanium is a solid metal. The atomic symbol of titanium is “Ti,” and Its atomic number is 22. Titanium has a higher density of 4.506 g/cm³, and its melting points and boiling points are 1,668°C (3,034°F), and 3,287°C (5,989°F). Titanium has a higher strength-to-weight ratio and higher corrosion resistance with considering the other metals.
Titanium has a specific chemical, physical, and atomic properties. Those properties are important for the titanium applications. Titanium is made with FeTiO3 and TiO2 using the Kroll method. Titanium production is an expensive process. Hence titanium is an expensive metal.
Titanium is available as 7 different types and 9 grades for different applications. These types and grades have various composition metals such as Aluminum, Vanadium, and Nickle. Due to these compositions, titanium characteristics have improved highly. Therefore titanium can use in a wide range of applications.
Titanium has main oxidation numbers such as +2, +3,+4. These oxidation numbers can make different titanium compounds. These compounds can be used in a wide range of applications. Titanium is not a rare metal in the earth. It is used in many applications and usages in different industries. Titanium has advantages and disadvantages. These should be considered when titanium is used.
What Are the Properties of Titanium?
Titanium is a metallic material. Titanium has 3 main types of properties such as physical properties, chemical properties and atomic properties. Each of these properties and its values are unique to the titanium. Titanium properties are very important when it is used in different applications such as solid metal to drill bit coating
What are the Chemical Properties of Titanium?
The chemical properties of titanium are the characteristics observed during a substance’s interaction with other matter, causing it to change its chemical composition. The significance of chemical properties are the ability to predict how a substance can behave under different conditions or when combined with others.
| Chemical Properties | Values |
|---|---|
| Atomic Number | 22 |
| Atomic Symbol | Ti |
| Atomic Mass | 47.867 amu |
| Electron Configuration | [Ar] 3d² 4s² |
| Group in Periodic Table | 4 |
| Period in Periodic Table | 4 |
| Electrons per Shell | 2, 8, 10, 2 |
| Common Compounds | TiO2 (titanium dioxide), TiCl4 (titanium tetrachloride), TiO (titanium monoxide) |
What are the Physical Properties of Titanium?
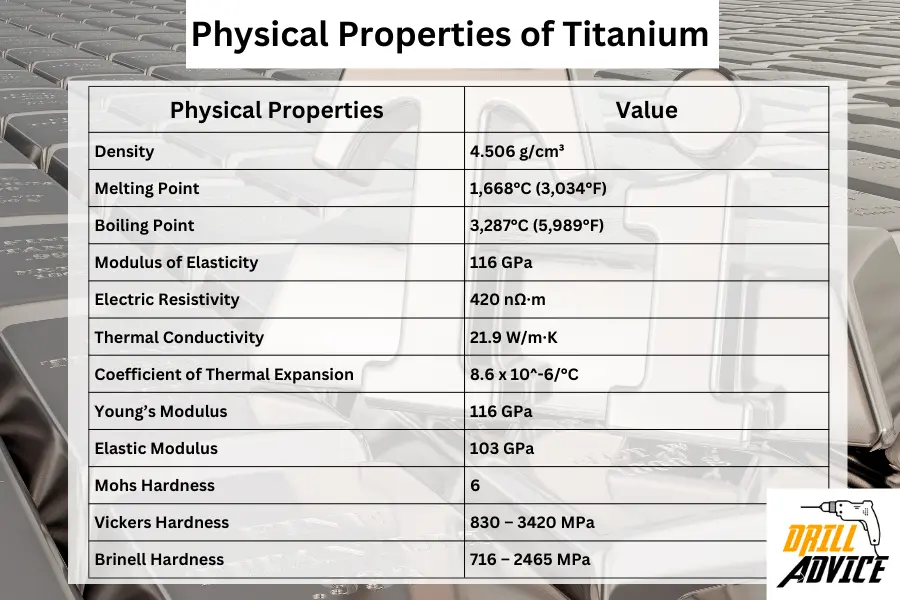
Physical properties of titanium are the qualities of the titanium that can be observed or measured without changing a substance’s chemical identity. These properties are important factors when it is applied in different uses. The important physical properties are density, melting point, boiling point, modulus of elasticity, thermal conductivity, and hardness properties.
| Physical Properties | Value |
|---|---|
| Density | 4.506 g/cm³ |
| Melting Point | 1,668°C (3,034°F) |
| Boiling Point | 3,287°C (5,989°F) |
| Modulus of Elasticity | 116 GPa |
| Electric Resistivity | 420 nΩ·m |
| Thermal Conductivity | 21.9 W/m·K |
| Coefficient of Thermal Expansion | 8.6 x 10^-6/°C |
| Young’s Modulus | 116 GPa |
| Elastic Modulus | 103 GPa |
| Mohs Hardness | 6 |
| Vickers Hardness | 830 – 3420 MPa |
| Brinell Hardness | 716 – 2465 MPa |
What Are the Atomic Properties of Titanium?
The atomic properties of the titanium are unique to the titanium atom. Atomic properties are based on the oxidation status and ionization energies.
| Atomic Properties | Values |
|---|---|
| Oxidation States | +2, +3, +4 |
| Ionization Energy 1 | 658.8 kJ/mol |
| Ionization Energy 2 | 1309.8 kJ/mol |
| Ionization Energy 3 | 2652.5 kJ/mol |
| Ionization Energy 4 | 4174.6 kJ/mol |
How is Titanium Made?
Titanium is made using ilmenite (FeTiO3) and rutile (TiO2). The method of titanium production is the Kroll process. That method was developed by William Kroll in the 1940s. Below are the steps of titanium production using the Kroll method.
- Step 1 – Ore Preparation
The first step is to extract the titanium-containing ore from the earth. The ore is then processed to remove impurities and to obtain a concentrate with a high titanium dioxide content. - Step – 2 – Chlorination
Then concentrated ore is subjected to a chlorination process, reacting with chlorine gas (Cl2) at high temperatures to produce titanium tetrachloride (TiCl4). This reaction can be represented as
TiO2 + 2 Cl2 + 2 C → TiCl4 + 2 CO - Step – 3 – TiCl4 Purification
The produced titanium tetrachloride is often impure and needs further purification. It is typically distilled to remove impurities such as iron chloride (FeCl4). - Step – 4 – Cl– Reduction
The purified titanium tetrachloride is then reduced to metallic titanium using a reducing agent, often magnesium (Mg), in a reactor. The reaction is exothermic and can be represented as:
TiCl4 + 2 Mg → Ti + 2 MgCl2 - Step – 5 – Separation
The metallic titanium is separated from the magnesium chloride byproduct through mechanical and chemical methods. - Step – 6 – Further Processing to Remove Impurities
The obtained titanium sponge (a porous mass of titanium metal) is often further processed to remove remaining impurities and to consolidate it into more usable forms such as ingots or billets. - Step – 7 – Alloying
In many applications, pure titanium may be alloyed with other elements to enhance its properties for specific uses. Common alloying elements include aluminum, vanadium, and nickel. These alloys will withstand 600°C plus temperature. - Step – 8 – Fabrication
The titanium can then be fabricated into various shapes and forms using processes like forging, rolling, machining, and welding.
The Kroll process is energy-intensive and involves several complex chemical reactions. As a result, the cost of producing titanium is relatively high compared to other metals.
What are the 9 Grades of Titanium?
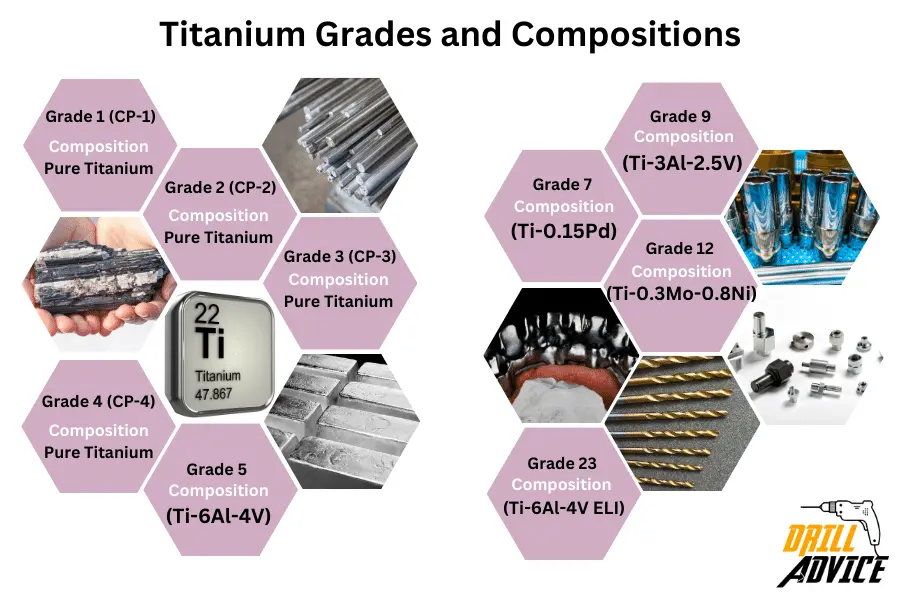
- Grade 1 (CP-1)
Composition materials – Pure Titanium
Characteristics – Ductile, soft (compare with other grades), excellent corrosion resistance
Applications – Used in chemical processing equipment and marine applications - Grade 2 (CP-2
Composition materials – Pure Titanium
Characteristics – Excellent corrosion resistance, formability, higher strength than grade 1
Applications – Used in chemical processing equipments, aerospace, medical, and chemical processing. - Grade 3 (CP-3)
Composition materials – Pure Titanium
Characteristics – Slightly improved corrosion resistance
Applications – Used for aggressive corrosion conditions - Grade 4 (CP-4)
Composition materials – Pure Titanium
Characteristics – More corrosion-resistant than grade 3
Applications – Used for chemical processing and marine environments. - Grade 5 (Ti-6Al-4V)
Composition materials – It contains 6% aluminium and 4% vanadium
Characteristics – high strength, toughness, and corrosion resistance
Applications – Used for aerospace, medical, and industrial applications. - Grade 7 (Ti-0.15Pd)
Composition materials – It contains 0.15% palladium
Characteristics – Higher corrosion resistance
Applications – Used in chemical processing and applications involving chloride-containing media - Grade 9 (Ti-3Al-2.5V)
Composition materials – It contains 3% aluminum and 2.5% vanadium
Characteristics – It has good weldability, moderate strength, and corrosion resistance
Applications – Used for aerospace, marine, and industrial applications. - Grade 12 (Ti-0.3Mo-0.8Ni)
Composition materials – It contains 3% aluminum and 2.5% vanadium
Characteristics – It has good weldability, moderate strength, and corrosion resistance
Applications – Used for chemical processing and marine applications - Grade 23 (Ti-6Al-4V ELI)
Composition materials – It contains 6% Al, 4% V (ELI) (This is the extra-low version of Grade 5)
Characteristics – It has good weldability, moderate strength, and corrosion resistance
Applications – Used for chemical processing and marine applications
What are the 7 Types of Titanium?
Titanium is categorized into 7 different types with considering strength, impurity percentage, corrosion resistance, formability, and weldability. Each of these types has different properties. Therefore they are used in different applications.
- Commercially Pure Titanium (CP Titanium): These are unalloyed titanium grades with varying levels of impurities. They offer good corrosion resistance, formability, and moderate strength. Common CP titanium grades include Grade 1, Grade 2, Grade 3, and Grade 4, each with different levels of oxygen content.
- Alpha Alloys: These alloys contain primarily alpha-phase titanium, which provides excellent high-temperature strength. Examples are grade 6 and grade 9 titanium
- Alpha-Beta Alloys: These alloys combine alpha and beta phases, offering a balance of strength, corrosion resistance, and weldability. One well-known alpha-beta alloy is grade 5, which is commonly used in aerospace applications.
- Beta Alloys: Beta alloys consist mainly of the beta phase and offer high strength along with good formability and weldability. Examples are grade 19 titanium.
- Specialty Alloys: These alloys are developed for specific applications and often contain additional elements to enhance certain properties. Examples are grade 19 for better corrosion resistance and Ti-grade 21 for elevated temperature performance.
- Intermetallic Compounds: Some advanced applications utilize intermetallic compounds of titanium, which exhibit unique properties such as high-temperature stability and oxidation resistance.
- Wrought vs. Cast Alloys: Titanium alloys can be further categorized as wrought (processed by rolling, forging, etc.) or cast (produced by casting methods). The processing method can impact the mechanical and physical properties of the material.
What are the Compounds and Applications of Titanium?
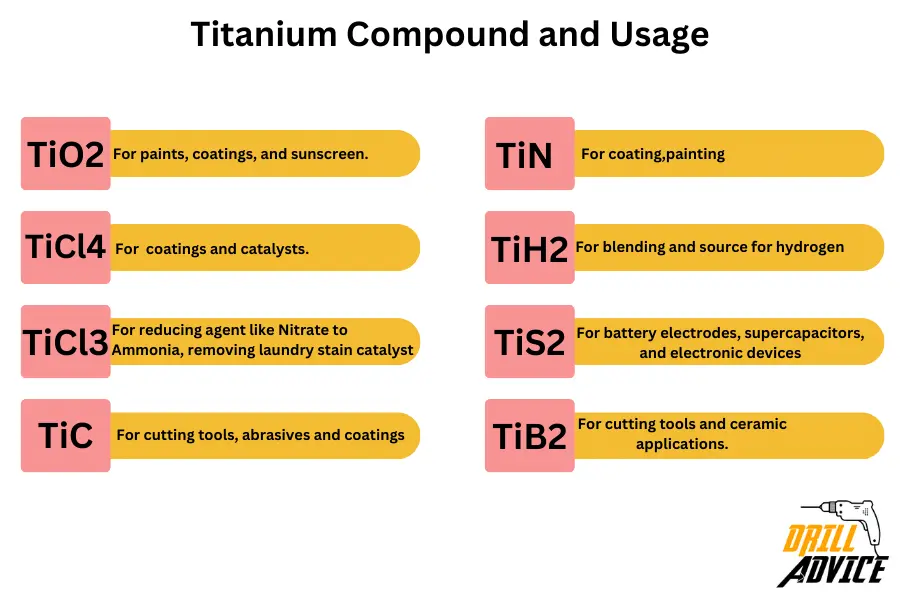
Titanium compounds are based on the +2, +3, +4 oxidation status. Due these various oxidation numbers titanium has mixed with other ions and makes different types of compounds. The most popular tinium compounds are TiO2, TiCl4, TiCl3,TiC, TiN, TiH2, TiS2 and TiB2. Each of these compounds has used for different applications.
- Titanium Dioxide (TiO2): TiO2 is a white pigment. TiO2 is commonly found in paints, coatings, and sunscreen.
- Titanium Tetrachloride (TiCl4): This compound is a key precursor in the production of titanium metal through the Kroll process. It’s also used in the production of titanium-containing coatings and as a catalyst in various chemical reactions.
- Titanium Trichloride (TiCl3): This compound is often used as a catalyst in the Ziegler-Natta polymerization process to produce certain types of polymers, including polyethylene and polypropylene.
- Titanium Carbide (TiC): A hard ceramic material. TiC is used as a component in cutting tools, abrasives, and coatings due to its high hardness and wear resistance.
- Titanium Nitride (TiN): TiN compound is known for its metallic golden appearance and is used as a hard coating for cutting tools, decorative items, and in electronics applications.
- Titanium Hydride (TiH2): TiH2 is used as a source of hydrogen in various industries, TiH2 is also employed in powder metallurgy to produce porous titanium materials.
- Titanium Sulfide (TiS2): TiS2 has semiconductor properties and is used in battery electrodes, supercapacitors, and electronic devices.
- Titanium Boride (TiB2): Known for its extreme hardness, TiB2 is used as an abrasive material in cutting tools and in some ceramic applications.
Is Titanium a Rare Metal?
Titanium is not a rare metal in the earth. Titanium is the 9th most available metal in the Earth’s crust. It is 0.57% by weight of the earth. Titanium is not able to find as a pure metal in the earth due to its strong tendency to react with other elements and form compounds. Most of the titanium is included in minerals such as ilmenite (FeTiO3) and rutile (TiO2) as an oxide form. Extracting pure titanium metal from these ores requires complex processes, which can contribute to its higher cost compared to some other metals.
Read More About – Black Oxide Coating: Types, Uses, Damages, Protect
What Are the Industries and Applications of Titanium?
Titanium is a strong metal, and it has higher corrosion resistance. Therefore titanium products are more durable than other metals. Hence titanium is used in the below industries in various applications and usages.
- Aerospace and Aviation: Titanium is used in aircraft components such as airframes, landing gear, engine parts, and fasteners to reduce weight while maintaining strength and durability.
- Medical Implants: Titanium is commonly used in medical implants such as artificial joints, bone plates, dental implants, and pacemaker casings. Because titanium doesn’t react negatively with the human body.
- Chemical Processing: Titanium is used in chemical processing equipment such as reactors, heat exchangers, and piping systems due to its resistance to corrosion,
- Marine and Naval Applications: Titanium is used in ship hulls, propellers, and other components exposed to saltwater because titanium has resistance to seawater.
- Power Generation: Titanium is employed in power generation systems, including nuclear reactors and gas turbine engines, where its high strength and corrosion resistance are crucial.
- Sports Equipment: Titanium is used in the production of sports equipment like bicycle frames, golf club heads, and tennis rackets due to its lightweight and durable properties.
- Automotive Industry: Titanium is used in exhaust systems, engine components, and suspension systems to reduce weight and improve performance.
- Architecture and Construction: Titanium is used in architectural applications for its aesthetic appeal, corrosion resistance, and durability. It’s often used for roofing, facades, and interior design elements.
- Jewelry and Accessories: Titanium has an attractive appearance. Titanium has a, lightweight nature, and hypoallergenic properties make it a popular choice for crafting jewelry, watches, eyewear frames, and other fashion accessories.
- Consumer Goods: Titanium is used in a variety of consumer products like smartphone cases, camping gear, and kitchen appliances due to its durability and aesthetic appeal
What are the 8 Disadvantages of Titanium?
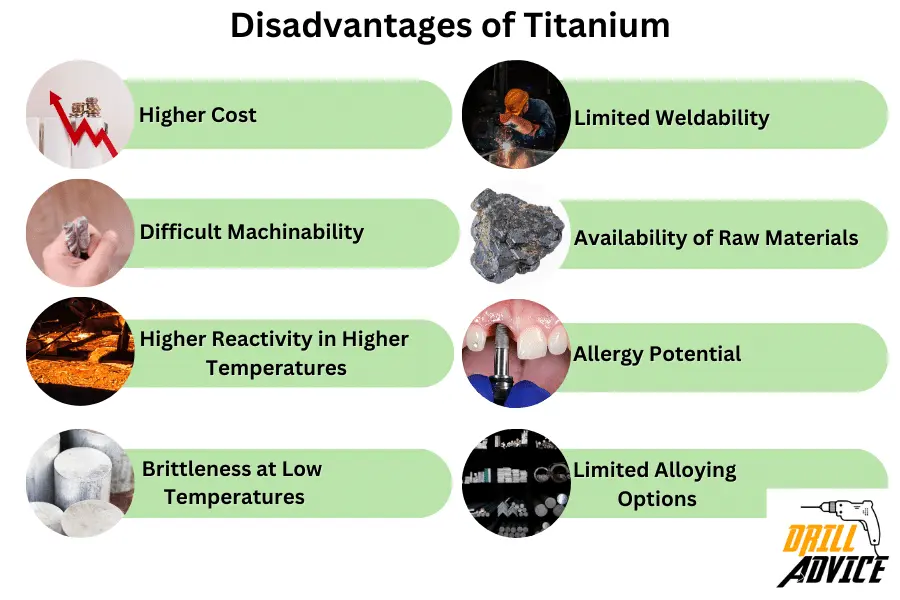
The disadvantages of titanium are based on titanium production, workability, less structural stability, and loose chemical bonds.
- Titanium is Higher Cost: Titanium production involves complex processes, contributing to its higher cost compared to other metals like steel and aluminum.
- Difficult Machinability: Titanium is known for its poor machinability. It tends to work-harden and can wear out tools quickly during machining processes.
- Higher Reactivity in Higher Temperatures: Titanium is highly reactive at elevated temperatures and can form undesirable compounds with elements present in its environment. This can limit its use in certain high-temperature applications.
- Brittleness at Low Temperatures: Titanium can become brittle at very low temperatures, which can limit its use in cryogenic applications.
- Limited Weldability: Welding titanium can be challenging due to its reactivity and sensitivity to oxygen and nitrogen contamination during the welding process.
- Availability of Raw Materials: While titanium is abundant in the Earth’s crust, its primary ores, such as ilmenite and rutile, need to be processed to extract the metal. This process can be resource-intensive.
- Allergy Potential: While titanium is generally considered biocompatible, there have been rare cases of allergic reactions to certain titanium alloys.
- Limited Alloying Options: Compared to some other metals, the range of elements that can be alloyed with titanium is relatively limited, which can impact its versatility in certain applications.
